Truth Science
Comprehensive clarification of how an experience is. Never final and what is accepted as fact today may be modified or discarded tomorrow. All about provability. Life is all about finding the right recipe

Matter
The body of information produced by chemical reactions and relationships in your psyche, mind epiphenomenon of matter

What is Mind? No Matter. What is Matter? Never Mind
George Berkeley

Nature
Creative life. Tears down, builds up. Forms patterns. NASA says the weight of the world is 5.972 x 10^24 kilograms. It’s entire 4.54 billion years of existence, it has always been the same weight. So what you put out there, stays out there

Orcam's Razor
Nature will often only use what is needed to accomplish the task

Energy
Neither created nor destroyed, it is universal. Finite. Flowing. Forever force. Polar. Waves. We Use, Make, Give and Take. Photosynthesis and our Food Cycle make molecules split apart to form energy to Grow, Move, Repair, and Reproduce. Like attracts like, similar energies attract each other. Sex and stress, both make and take energy
Universal Energy: (Chi/Prana) Subtly flowing through and present in all living things (Aura/Soul) connected to the past, present and future (soul essence) connects us to universe. Everything in the universe exists in a state of constant vibration. The morphogenic field concept calls it a cosmic library of all experiences and memories of human minds from their physical lifetime. Energetic pathways or meridian lines (Chakra's) align w/ vibrational frequencies of the universe itself. Psyche is our interface between invisible universal flow of energy and the manifest world. Psychic power is the ability to tap into the cosmic field of flowing energies across different planes (Web of interlocking Energy Waves). Albert Einstein's Quantum fields worked with manipulating invisible energy fields. Channeling (mind) universal energy and directing life force energy (L-field) (Lunar cycles) electromagnetic field of any organism. Thoughts and feelings have their own vibrational frequencies and generate their own level of energy. Emotions emitted. Feelings flow (shape plus change/motion plus form)
Human Energy:
1) Combustion
2) Respiration
3) 10% from the Sun
Renewable Energy: Generate electrons
1) Wind
2) Water
3) Geothermal
4) Biofuel
Energy Moves in and out of Earth's 4 Physical Systems:
1) Atmosphere
2) Hydrosphere
3) Lithosphere
4) Biosphere
Can be Energy:
1) Sound
2) Light
3) Sex (source)
Potential Energy: Stored energy. Exists in possibility
1) Chemical
2) Elastic
3) Nuclear
4) Gravitational
Kinetic Energy: Energy of movement
1) Thermal
2) Mechanical
3) Electrical
4) Magnetic
Electricity: Natural phenomenon, serviceable energy when harnessed and directed
Electromagnetic Spectrum: Non Ionizing (waves) radiation to Ionizing radiation (rays) in the middle of the scope is visible light, separated by Hertz class (Gamma, Alpha, Beta, Theta, Delta)

Chemical Influences
In natural systems energy exists on 2 levels
1) Macroscales: Everyday objects can be counted and measured
2) Microscales: Countless atoms swim in random motion, generation electrons
The meeting of 2 atoms, where if there is any reaction, they both are transformed. You can exert no influence though if you are not susceptible to influence, open and willing to meet, give and take, to have. Usually you get back, what you put out there. Elements of human personality, bonding with others. Synthesizing
Bond Length: Relationship or distance between 2 nuclei at the point of minimum energy, where attractive and repulsive forces cancel out
Nature of the Bond: Between atoms related to the distance between them or how positive or negative they are
Electronegativity: The ability of an atom to attract/share electrons. Strength of the atoms bonds
Like Charges: Repelled or stressed out by the attractive force and tries to relieve the stress by getting closer, this pull is so strong that the stress level or energy rises when the 2 are separated. Too similar to each other
Proper Orientation: Needed to be aimed at each other, or particles won't react. Lives must align and illicit enough energy, either negative or positive to disrupt electron clouds
Collision Theory: How chemical reactions occur and how certain factors affect the rate of those reactions, the probabilities of interacting
1) Velocity of the colliding particles
2) Their energy
3) Specific chemical configuration. No reaction will take place unless the particles are properly oriented toward each other
Activation Energy: Collision energy required for chemical reactions or the amount of energy needed to arouse and disrupt the stable electronic configuration of any specific molecule so that the electrons can be rearranged. Heat is kinetic energy
Pressure: Increases when reactants are more concentrated or a decreased distance between molecules, on top of each other
Valance: Combining capacity of an atom. Extra or missing electron in the outermost orbital of the electron cloud. Ability to give and take. Law of Attraction
Polarities: Orbital configuration determine the shape of the molecules and the shape of the molecules determines how they will behave, what forms they take and what properties they have. For polarity to occur in a molecule, there has to be a dipole moment: separation of the charge around the molecule into a more positive area and a more negative area. Polar molecules are ionic. They are usually asymmetrical in shape. Liquids. Good at dissolving solids that are composed of polar or ionic compounds. Charged particles. Electrolytes. Polarizing personalities
Compounds: 2 or more different elements bonded. Ex. H2O, CO2. CH4
Anabolic: Enzyme constructs and consumes, using energy to build macromolecules by combining simpler molecules
Catabolic: Enzyme, breaks and releases macromolecules into simpler components, releasing energy. Polar
Ionization: Charged Particle. Balancing H+: Acid/Base reaction. Acid dissociates, making 1 or more H+. Base binds into 1 or more OH- excepts H+
Ionic Bonds: Polar molecule (H2O) solvent separates/dissociates. Ionic bond (solutes) sugars and salts. High difference of electronegativity. Vulnerable. High electronegativity
Non-Polar Covalent: Symmetrical, share. Zero electronegativity
Polar Covalent: H2O has a bent shape. Low electronegativity

Metabolism
Total chemical reaction: Pathway series of chemical reactions that aid in the break down of complex molecules. Essential to life, synthesizing life sustaining substances. Sum of the process in the build up and destruction of protoplasm
Catalyst: Substances that can speed up a chemical reaction. someone who sets up change, but isn't affected by the change
Enzyme: Increase reaction rate with increasing temperature, breaks H+ bonds, everything in the cell happens because of them
Substrate: Base in which an organism lives. Substance enzymes act upon
Enzymatic Action:
1) Substrate contacts the active site on enzyme
2) Forms enzyme-substrate complex
3) Substrate transformed into products
4) Products released
5) Enzyme recovered unchanged
Bacteria Metabolism: Taught molecular biologist how the "message" in DNA is transcribed into mRNA and then translated into amino acid sequence of proteins

Atom and Mitochondrial Eve

97% of the same 11 Elements that make a star, make the human body's 11 systems
We are the star people, sharing the same 5 point shape, and made with the same 11 Ingredients:
1) Oxygen
2) Carbon
3) Hydrogen
4) Nitrogen
5) Calcium
6) Phosphorous
7) Potassium
8) Sulfur
9) Sodium
10) Chlorine
11) Magnesium
These ingredients are all elements and are the purest form of matter, unable to be broken down to simpler substances, it contains 1 molecule or 1 type of atom. The atom is the smallest unit of an element. The basic building block of all matter. Always here, always moving, and always making and breaking bonds, doing whatever possible to reduce their overall energy. The lowest energy is reached by a balance between the atom's repulsive and attractive forces. Ions or electrolytes are atoms with charged particles. Neutrons are neutrally charged subatomic particles, they are attracted to the positively charged protons and hold together within nucleus's strong nuclear force. Hydrogen with it's 1 proton does not need any neutrons to be stable. Negatively charged electron clouds surround the nucleus. 2, 8, 8 and 18 are the 4 energy levels and probability distribution of electrons forming orbits or fixed paths electrons revolve in
Oxygen (8)
Key component of cell division. Bacteria growth relies on diffusion or the movement of oxygen and nutrients with protoplasm. Oxygen deprivation of 8 minutes cause cells to starve and die
Valence: 6 Max Bonds: 2
Carbon (6)
Skeletons. Organic Compounds 1) OH: Lipids, Carbs 2) CH3: Methly forms DNA, Energy, Metabolism, Structural Organic Molecules
Valence: 4 Max Bond: 4
Hydrogen (1)
Form many weak bonds, affected by heat. Free hydrogen change the shapes and functions of enzymes and hormones. Cause change in distributing of ions or electrolytes. Change the excitability of membranes. Acid/Base balance
Valence:1 Max Bonds: 1
Nitrogen
Amino Group (NH). Foods (increase protein) long chains of polypeptides producing protein synthesis. CO2N: amino acids
Valence: 5 Max Bonds: 3
Calcium (Electrolyte)
Behaves the opposite of Phosphorous and Magnesium. Desired levels of Calcium (2.2-2.6)
Excessive Levels: Parathyroidism, Excessive Vitamin D, Renal Disease (decreased excretion), Neuromuscular excitability, Arrhythmias, Cardiac Arrest, Weakness, Confusion, Stupor, Stones, Nausea
Decreased Levels: Burns, Low Vitamin D, Renal Tubular Disease and Failure, Depressed Bone
Phosphorous (Electrolyte)
Builds bones and teeth. Small amount in food. Tries to be like Magnesium. Acts the opposite of Calcium. ATP and DNA collect energy. Backbone of DNA. Has the ability to form many possible bonds. Hydrophilic and Hydrophobic. Cellular Membrane
Desired Levels: (.8-1.5)
Excessive Levels: Renal Failure (Decreased Urination), Increased GI Absorption, Decrease Parathyroidism
Valence: 5 Max Bonds: 5
Potassium (Electrolyte)
Desired Levels: (3.5-5)
Excessive Levels: Renal Failure, Decreased Aldosterone, Burns, Nausea, Diarrhea, Bradycardia, Heart Arrythmias, Skeletal/Muscle Weakness, Low Blood Pressure, Tight/Contractures.
Decreased Levels: From GI suctioning, Cushings, Starvation, Increased Aldosterone, Diuretics, Heart Arrythmias, Metabolic Alkalosis, Mental Confusion, Vomiting
Valence: 1 Max Bonds: 1
Sulfur
Insulin Production. Making Protein. Regulating Gene Expression. Building and Repairing DNA. Helps Body Metabolize Food. Protect from cellular damage and oxidative stress. Energy metabolism and protein structures
Sodium (Electrolyte)
Behaves the opposite of Potassium
Desired Levels: (135-145)
Excessive Levels: Dehydration, Lethargy, Confusion, Irritable, Flush, Edema, Fever, Pernicious Anemia B12
Decreased Levels: Water Retention, Solute Loss, Vomiting, Burns, Gastric Suctioning, Decreased Aldosterone, Neurological Dysfunction, Excessive Water Intake, Low Blood Pressure (circulatory shock)
Chloride (Electrolyte)
Tries to be like Sodium. Kidneys produce Chloride and excrete free Hydrogen, binds to Phosphate and Nitroge Desired Levels: (95-105)
Excessive Levels: Dehydration, Urinary Retention, Metabolic Acidosis, Parathyroidism, Decreased pH Decreased Levels: Metabolic Alkalosis, Vomiting, Decreased Aldosterone, Increased PH
Magnesium (Electrolyte)
Desired Levels: (1.5-2)
Excessive Levels: Rare, Antacids, Renal Failure, Lethargy, Decreased CNS, Decreased Respirations, Cardiac Arrest
Decreased Levels: Alcoholism, Diarrhea, Malnutrition, Diuretic, Tremors, Muscle Tetany, Convulsions
Acids, Bases and Salts

PH
Balance of Acids/Bases. Building and breaking H+ bonds. Human pH (7.35-7.45) Bacteria thrives between (6.5-7.5)
pH Scale:
(1) Stomach Acid A
(2) Lemon juice C
(3) Grapefruit I
(4) Tomato D
(5-6) Urine S
(7) Milk, Blood, Water &
(8) Sea Water B
(9-10) Milk of Magnesia A
(11-12) Bleach S
(13) Oven Cleaner E
(14) Lime Water S
Acid
Hypertonic. Falling apart. K+, Ca+, Mg, CO2, H2CO3 (carbonic acid)
Acidosis: Decreased bowel sounds, bradycardia, constipation, flaccid, hyporeflexia, bradypnea, hypotension
Hyperkalemia: Tachycardia, increased urine output
Base
Bulking. Potassium decreases and makes less insulin. Energy consuming. NaOH, NH3. Ph. Na. Cl. HCO3. Kidneys dissociate (HCO3 + H) or bicarbonate and hydrogen
Alkalosis: tachycardia, tachypnea, hypertension, seizures, hyperreflexia, hypokalemia, decreased heart rate, decreased urine output
Salt
Polar covalent. H2O. Cell Cytosol: Na+, K+, Cl-, HCO3. Albumin: blood buffer. Metabolism (H2O + CO2) Free H+, change shape and function of enzyme and hormones, causing change in distribution of ions or electrolytes, leading to a change of excitability of membranes
Dissociate: Acids, bases, salts into H+ or OH-
Molecules
Molecular Self-Organization: Fundamental units of information translated in linear and unique fashion into proteins that act as biology's molecular work horses, carrying your book of life
Organic Organization: Living beings acquire structure and coordinate among their component molecular parts
Body:
1) Proteins (16%)
2) Minerals (4%)
3) Fats (16%)
4) Carbs (1%)
5) Water (64%)
Biological Molecules:
1) Instructions
2) Energy Storage
3) Sources of Energy
Carbs, Lipids, Proteins, Nucleic Acids. Different by proportions of the same elements
Biological Absorption:
Mouth: Fats, Carbs
Liver: Fats
Stomach: Proteins, Fats
Pancreas: Fats, Carbs, Proteins, Nucleic Acids
Small Intestine: Carbs, Proteins, Nucleic Acids
Waste Created: Carbs (CO2). Proteins (sulfuric acid). Fats (fatty acids) Ketones break down fat for energy
Glucose Regulation: Brain needs 120 grams of glucose (60-70%) of what we eat, or 2 M&Ms. Most basic simple sugar and ingredient of ATP, which is able to make energy anywhere in the body, passing through blood brain barrier. Disacharides are a pair of sugar monomers, COH maltose= glucose + glucose) Ex. Sucrorose= Glucose + Fructose. Polysacharides are many sugar monomers joined. Ex Cellulose, Starch, Glycogen (chains of glucose) Plant cell wall, long chain of sugar units (starches) glycogen stored in liver. Monosachrides simple sugars (glucose/fructose) metabolic fuel
ATP: Carbs (sugars). Fats (glycerol/fatty acids). Proteins (amino acids). Energy coupling for the body's muscles and nerves. Removes acid from the body
Carbs: Glucose. ATP. Catabolic enzyme, breaks and release. Main source of energy found in blood and stored in the liver and muscles for future energy needs. Liver turns in fat tissues. (C+, H+, O+) Covalently bind to proteins or lipids (glycolipids) in membrane and form desmosomes (cell to cell adhesion sites (barcode) seperating and blocking itself from the other cells and foreign bodies
Na+ K+ Pump: Moving Na+ and K+ ions across the cell membrane. Na+ and H2O pumped out and K+ pulled in. Active transport using ATP for energy
Adipose Cell: Fat storage cells, insulate/temperature. Stores 2x energy than carbs do
Saturated Fats: C+ and H+ with out double bonds and kinks. Ex. butter melts in warmer temperatures
Unsaturated Fats: Not all C+ and H+. Double bonds and kinks. Ex. Corn/Peanut oil keeps fat solidifying at lower temperatures
Trans Fats: Partially H+ on otherside double bonds, fewer kinks
Lipids: (C+, H+, O+) Main component of cell membrane. Fatty acids, Fats. Store Energy. Myelin. Triglycerides. Phospholipids. Fruits. Nuts. Seeds. Plants. Fatty acid is a simple lipid of ( C+, H+) chains that influence viscosity. Hydrophobic, water doesn't adhere to it and doesn't mix
Cholesterol: Interior membrane, it stabilizes and strengthens. Nerve cells have high cholesterol limits, giving the ability to let cells in and out. Ex. Digestion
Steroid: Considered lipids, hydrophobic. Usually big Common 4 ring structure with ( C+) Keeps membrane pliant at low temperatures. Cholesterol. Estrogen
Proteins:
1) Albumin (54%)
2) Globulins (38%)
3) Fibrogen (4%)
4) Prothrombin (1%)
Protein: Macromolecules, passenger, performing most of the body's tasks. Structure, signal, transport, regulatory enzymes, peptide bonds of amino acids (at least 50 bonds). 100,000 different proteins exist in the body. Cells need enzymes, hormones, antibodies, hemoglobin to function. Most of solid materials in body (structural and functional activities). Muscle tissues (myosin)
Channel Proteins: Facilitate. Transports chemicals in/out of cell
Carrier Proteins: Bind to a molecule through membrane. Communicates and responds to signals. Metabolize. Anabolic enzymes constructs and consumes energy
Amino Acids: Subunits of proteins (carboxyl acid) ionic covalent bonds cause variable side influencing protein shape, charge, and hydrophobicity
Protein Structures:
1) Primary: Amino acid sequence
2) Secondary: Helixes and pleated sheets
3) Tertiary: 3D folding of one polypeptide
4) Quaternary: 2 or more folded polypeptides
Membrane Proteins:
1) Transport
2) Receptors for signal transduction
3) Attachment to cytoskeleton and extracellular matrix
Conjugated Proteins: Proteins attached with
1) Carb
2) Lipid
3) Nucleic Acid
Denaturation: Protein unfolds, or misfolds like in Sickle Cell. Heat involved
Nucleic Acids: Complex compounds making DNA and RNA. Bonds of (C+, H+, N, O, Ph) making cells and their organelles

Vitamins and Minerals
Minerals: Iron, Hemoglobin
Vitamins: Made up of the same elements
1) Carbon
2) Hydrogen
3) Oxygen
4) Nitrogen
5) Cobalt
Arranged differently performing 1 or more specific body function. Compounds needed to prevent diseases (deficiencies) Classified by solubility or Fat and Water soluble. Fat soluble are stored in the body and include Vitamin A,D,E,K. Water soluble aren't stored in the body and are Vitamin C,B
Vitamin A: Protein synthesis, immune function, forming collagen, epithelium maintenance, night vision, pressure ulcer. Form and maintain skin, hair, and proper bone growth, teeth. Immune maintenance of protective linings (lungs, intestines, urinary tract) Normal reproduction, protects against certain forms of cancer. Vision protects cornea from becoming cloudy, dry and the ability to adjust to light. Beta carotene converted to vitamin A and makes food orange
Hyper Vitamin A: Dry, scaly skin, bone pain, soreness, stunted growth, liver damage, nausea and diarrhea
Vitamin D: Also made by body and acts like a hormone (chemical messenger) maintains blood calcium and phosphorous levels. UV rays on skin converts cholesterol like compound into vitamin D precursor in blood
Hypo vitamin D: Rickets, scoliosis, osteomalacia, osteoporosis, hypertension, cancer dementia, auto immune disorders
Hyper vitamin D: Nausea, vomiting, diarrhea, fatigue, thirst. Calcium deposits in heart and kidneys. With Vitamin E may help against dementia and Parkinson's
Vitamin E: Fat metabolism, collagen synthesis, cell stability, antioxidant prevents oxygen from destroying important substances by combining with oxygen molecules so they don't oxidize or destroy the cell. Slows down natural aging process and protection against cancer. Cell health (blood) Metabolism of Vitamin A and immune functions. Plant foods, vegetable oil, margarine, wheat germ. Prevents oil from going rancid
Vitamin K: Produces protein involved in clotting and calcium metabolism. Made in body by bacteria in the intestines which usually is enough for the body. Green leafy vegetables, liver, milk, eggs
B Complex: Works in every cell in the body, function as coenzymes activating enzymes, which boost chemical reactions supporting an array of body functions, like excreting urine
Vitamin B: Deficiency is rare. Key roles as coenzymes in energy metabolism. Enzymes (specialized proteins that speed up specific chemical reactions, help release energy from glucose, fatty acids, and amino acids
B1) Thiamine: Poor appetite, depression, confusion, weakness, muscle wasting, heart problems, decreased nervous system function
B2) Riboflavin: Low: Cracks in the corner of mouth, skin rash, poor healing, burning itchy eyes. Skin, eye function. Found in milk, whole grains, organ meat B3) Niacin: Maintain skin, nervous system, digestive tract, organ meat, poultry, fish, complete protein, milk, eggs, tryptophan Low: fatigue, poor appetite, indigestion, skin rash, chronic (pellagra)
High: Flushing, rash, tingles, hives, nausea, diarrhea, abd discomfort, liver malfunction, hyperglycemia, abnormal rhythm. Whole grain. Rebuilding energy production, converts glucose, fatty acids, fats, immune maintenance, RBC formation
B6) Pyridoxine: Coenzyme with carbs, fats and especially protein metabolism, where its used to make RBCs and convert amino acidtryptophan in niacin (B3) Meat, fish, poultry, potato, banana, brocoli, spinach. Low: muscle twitch, rash, greasy skin and small cell anemia B9) Folate: part of coenzyme that makes new cells like RBCs, WBCs and digestive tract cells
Low: Megaloblastic anemia (GI, pregnant birth defects (neural tube deficits) or brain and spinal issues. B12) Cobalamin: Present in every cell and folate. Involved with growth of every new cell. Protective cover around nerve fibers. Only in animal products, vegan issues. Intrinsic factor is needed for absorbing B12 produced in the stomach, pernicious anemia
Vitamin C: Tissue repair. Regenerates forms collagen. Metabolism of amino acids. Helps absorb iron and activate copper. Stress, burns. Collagen (Protein that strengthens/supports bones/teeth, muscles, cartilage, blood vessels and skin tissue. Increased resistance of infections. Prevents oxidation of vitamin A and polyunsaturated fatty acids in intestines. Widely additive to foods (preserves freshness) labeled as sodium ascorbate, calcium ascorbate, ascorbic acid. Fruits, citrus, tomatoes, strawberries, cantaloupe, green and yellow vegetables. Increased need situations are pregnancy, nursing, growth, fevers, infections, burns, fractures, surgery, cancer

Genetics
Passage of knowledge to generations, molecular memory. Geneticist adapted the lexicon of cybernetic system theory, which was developed to understand how complex technological systems found in electronic engineering; robotics and communication. Different component in genome linked into circuits and regulated by feedback loops and switches, as they pass signals from one unit to another. Genomes perform their role of organizing and regulating life in a modular and hierarchal fashion. Electronic components connected into basic circuit element. Amplifiers or logic devices in turn arrange
Gene Expression: Effective procedure or anything that can be solved in prescribed # of steps
Messenger RNA: Passes on genetic information (DNA to Protein)
Ribosomal RNA: Part of the ribosomes that synthesize proteins as directed in the messenger RNA
Genetic Sequence of DNA: Matter of information, barcode, digital code, molecular memory producing proteins, producing life, tiny molecules regulating cells nucleic acid (DNA)
Genetic Circuitory: Signal rate of synthesis of a protein. Digital code to your cookbook. 25,000-30,000 genes
RNA Splicing: Create Messenger mRNA
Transfer RNA: tRNA combines 20 different amino acids. Anti-codon. Transfers amino acids for growing proteins
Recombination Frequency: % of recessive offspring
Polygenetic Inheritance: Skin/height. Character. Inheritable factors
Hereditary: Transmission of traits (alleles)
Dominant: Determines organisms appearance
Recessive: Not noticeable
Loci: Specific location of a gene along a chromosome
Phenotype: Appearance or expression of the gene's traits
Genotype: Genetic makeup of a trait, genes of an organism
Sex Chromosomes: X and Y. XX = Girl. XY = Boy

DNA
Redesigning Life like computer engineering, a question of coding and circuit design. Genetic information acting as protein workers. Vehicle of genes passing instructions down generation lines. Information encoded in digital sequence of molecular building blocks along it's helix, mechanisms by which the information could be copied during replication. By the 1970s, started modifying instructions for Life by using natural enzymes to edit and paste portions of recombinant DNA, thinking life as a form of engineering. Design. Living factories
1) Contains like a barcode found in order of bases. Double nucleotides (A and T, T and A, G and C, C and G)
2) DNA transfers code to messenger RNA molecule, which doesn't contain Thymine but Uracil protein that unzips rung of DNA, transcription begins
2.1) Promoter (base sequence pair)
2.2) Ends with terminator
2.3) Detaches from DNA and continues to reassemble 3 groups of nucleotides in RNA codon (UACG) code for 64 different amino acids but mostly only 20 are used
3) mRNA leaves nucleus and attaches to a ribosome, in the cytoplasm and attaches to tRNA
4) tRNA: sequence of anticodons that pair with mRNA codons bringing amino acids to ribosomes, unpaired nucleotides
5) Amino acids link together anticodon (CGG) and (GCC) and brings Alanine to bind with 2nd METH and the third amino acid tRNA with Tryptophan attaches anti-codon (ACC) with amino acid Threonine and binds to Tryptophan to build a protein
6) Ribosome releases protein, building translations of 50 amino acids linking to form proteins
Finding the Right Recipe:
1) Gene recipe to protein
2) Gene for protein is part of the DNA molecule "cookbook"
3) mRNA writes down recipe and carries it back to cytoplasm "kitchen"
4) Protein "finished product", amino acids brought by tRNA "ingredients of protein"
Gene Activation: The how to know when to unzip, maintaining homeostasis
DNA Polymerase: Flow of genetic information, transcribing RNA
1) DNA
2) RNA
3) Proteins
4) Links to genes
Transcript: Synthesis of RNA under direction DNA. Making RNA
1) mRNA
2) tRNA
3) rRNA. Regular, linear fashion from one nucleotide base to the next in bacteria/virus. Organism with nucleus its an entires base sequence of DNA that
1) Transcribed in =to mRNA
2) RNA gets modified by eliminating parts of it and splicing the rest together
3) Final "mature" mRNA gets translated into proteins
Translation: Synthesis of proteins under RNA proteins. Amino acids. Subunits of protein. 20 naturally occurring
Codon: Stop 3 letter combo (UAA, UGA, UAG)
Exon: In gene. Splice out/unzip makes proteins mRNA. Portion that gets translated into final proteins
Intron: Don't go in. Segment of DNA with genes that dont make proteins. Portion of oringinal DNA sequence that gets edited out in the process
Chromosome: String of DNA. Polymer of nucleotides. Physically carries hereditary information (genome) all the genetic information in a cell. Consist of genes
Genetics:
1) Study of what genes are
2) How they carry information
3) How the information is expressed
4) How genes are replicated
Gene: Segment of DNA that encodes a functional product, usually a protein
DNA expression: Genetic information used within cell to produce proteins needed for the cell to function
Replicant: Genetic information transferred between generations of cells. Vertical gene transfer
Recombinant: Genetic information transferred between cells of same generation. Horizontal gene transfer
Regulation: Constitutive genes expressed at fixed rates. Other genes expressed only as needed
Repressible Operon: Regulatory gene Operon: On
Repressor: Off and transcription and translation continues
Mutation: Genetic material change for
1) Harmful
2) Beneficial
3) Neutral. Radiation/Ionizing (x-rays - gamma=rays) cause formation of ions that can react with nucleotides and deoxyribose-phosphate backbone
Chromosomal Types: Autosomes
1) Telocentric
2) Acrocentric
3) Submetacentric
4) Metacentric
Sex chromosomes: X and Y. 46 diploid chromosomes = 23 homologous

Cell Corporation

Cell
1st discovered by Rob Hooke in 1665. It is the smallest living unit
Cell Theory: All living things are composed of cells and all cells come from other cells
Proliferation: Reproduction of new cells (cell growth/division)
Differentation: Acquisition of functions differing from size/shape or original cell. Processes more or less specialized
Morphology: Structure of cell
Coding: Surveilance. Genetic mutation and protein expression. Cell membrane
Cell Membrane: Surrounds all cell. Secrete chemicals causing action potentials, reducing polarity of the membrane, causing depolarization. Selectively permeable, allowing certain types of material to pass through
1) Passive Transport: No energy for diffusion passing molecules from high concentration to low. Ex. Water molecules and sugar molecules or osmosis
2) Active Transport
Organelles
"Different Departments" The number of organelles, determines the cell's purpose
Mitochondria
"Power House" of the cell. Builds/Synthesizes molecule of ATP for cellular energy. Contains some DNA and ribosomes and replicates itself separate of cell's DNA. Existed as own in bacteria. Mother's mitochondria single eve, in Africa 200,000 years ago. Human blood cells contain only 1 mitochondria, while there are 1000s in the liver cells
Nucleus
Double Membrane (Nuclear envelope) inner part of the envelope is smooth. Control center. Not found in blood cells. Specialized in nerve cells
Chromosomes
Protein molecules attached to DNA. String of DNA. Polymer of nucleotides. Physically carries hereditary information (genome) all the genetic information in a cell. Consists of genes. Prokaryotic chromosomes carry information for
1) Amino Acid metabolism
2) DNA replication and repair
3) Lipid metabolism
4) Carbohydrate metabolism
5) Membrane synthesis. Maintaining Chromosome structure and control the activivity of its genes based on the number of proteins. Chromatin is equal parts DNA and Protein. A string of DNA, consists of 3 proteins that form double helix rung together with base molecules
Vessicle
Pinch of golgi aparatus that travel to cell membrane, where chemicals are released. Nervous system has tons of them
Lysosome: Special type of vessicle (package) with digestive enzymes (waste department) breaks down
1)Proteins
2) Lipids
3) Nucleic Acids
4) Carbs and digests defective organelles
Perioxisome: Contain enzyme used for energy metabolism (Turbine) in the power department
Chromatin Granules
Present in the cell's nucleus. Threads of DNA containing genes containing information to make types of proteins used for directing cell activity (various activations)
Cell Membrane
Protoplasm: Every structure that makes up the cell membrane
Cytoplasm: Nucleus/inside cell
Cytoskeleton: Structural beams, corridors "scaffolding" Ex. muscle, 3D structure made of 3 protein filaments ( thread like protein fibers)
1) Actin
2) Intermediate (flexing)
3) Microtubles movie organelle around cell and framework
Cilia: Moves fluids and other substances past cell Ex. mucus clears throat
Flagella: Moves the cell Ex. sperm
Endoplasmic Reticulum
“Highway System” series of tubes in cell‘s cytoplasm that allow material to move around cell
1) Smooth ER: makes lipids and carbs molecules
2) Rough ER: covered in Ribosomes, builds protein packages
Ribosomes
“Workers” produced by RNA, they carry out the code for protein production the mitochondria needs. Following orders and assembling genes in 2 chromosomes before cell division. Factory machine, building
Golgi
Stacks of proteins, cut down to smaller hormones. Pores allow large molecules unable to diffuse through, to travel in and out of membrane
Golgi Apparatus: "Post Office" packaging department. Modifies and releases and packages neurotransmitters
Cellular Regulation
All functions carried out within a cell to maintain homeostasis, responding to extracellular signals (hormones, cytokines, neurotransmitters) and produces intracellular response (replication, growth)
People Cell:
1) Proteins (7%)
2) Water (91%)
3) Solutes (2%)
Cell Division: (1, 2, 4, 8, 16, 32, 64...) 3, 6 and 9 exist on completely different plane
Cellular Respiration: Osmosis. Exerginic energy release, cells extract energy from the transfer of electrons. Glucose and O2. 8 minutes without O2, lysis begins
Isotonic: Blood plasma
Hypotonic: Solutes concentration increased inside cell. H2O rushes in. Decreased osmotic pressure. Depressed
Hypertonic: Solutes concentration decreased outside cell. H2O rushes out. Increased osmotic pressure
Dehydration Synthesis: Condensation, where molecules join and produce water. C6H12O6 (glucose) and C6H12O6 (fructose) = H2O
Hydrolysis: Molecules split and water used C12H22O4 (sucrose) + H2O
Extracellular Fluid: 40L of H2O (65% of body's weight)
Intracellular Fluid: K+, Mg+, HPO4, protein, SO4
Interstial Fluid: Na+, HCO3, Cl-
Plasma: Na+, HCO3, Cl-, Protein
Endocytosis: Transport requires energy. Membrane surrounds material to get inside cell. Ex. WBC
Exocytosis: Transport requires energy. Material inside cell, packaged together (vessicle) Fuses with membrane and releases contents out of the cell. Ex Releasing waste and bringing in nutrients
Facilitated: Needs help getting through membrane
Simple Active: Low to high. Ex. Food giving energy back
Amoeba: Single cell organism, that asexually reproduces with mitosis
Meiosis: Order of bases determines inherited characteristics (fertilized egg cell) Haploid reunite to make new diploid. Alternating generations. 2 pairs of chromosomes
1) Duplication of genetic material (mitotic) division. Chromosomes duplicate homologous pairs that find/line up and share. Mitosis daughter cell (46 chromosomes)
2) Nuclear envelope fades and homologous chromosomes unite
3) Replicate (4 sister chromatids connect and make strands of the chromatid material) 92 chromatids crossover
4) Reduces 46 chromatids and new nuclear envelope surrounds chromatids 5) Envelope disappears and 2ndary spermatocytes divide to 4 spermatids (23 chromosomes)
Spermatoza: Active sperm cell
Eggs: Have similar process (polar body disintigrates) Mature egg formed 6 weeks of conception
Cell that do not divide:
1) RBCs (marrow continues to replace)
2) Mature skeletal, cardiac and nerve cells
3) Muscle/adipose grows/shrinks
Mitotic: Cell physically divides
1) Mitosis (nucleus and contents duplicating chromosomes) divide/duplicate into 2 daughter nuclei
2) Cytokinesis (begins before mitosis ends) cytoplasm divides to produce 2 daughter cells. Division stage
Cell cycle: Phase of growth and division, formation and division
Interphase: High cell metabolic activities (enzyme) growing stage. Doubling everything in the cytoplasm and duplicating DNA
Horizontal gene transfer: Between cells of same generation
Genetic Recombination: Exchange of genes between 2 DNA molecules. Crossing over when 2 chromosomes break and rejoin

Virus
Virus: Nucleic acid surrounded by protein envelope that invades cells and acts like a metabolic machine to reproduce itself. Interferons are proteins released by the body when a virus has made entry, and signal to stop the invaders replication. A virus needs a host to survive, they affect all forms of life, and mutate quickly. Viral genes continue to be around longer than actual virus, hijacking DNA to reproduce more of itself
Retro Virus: DNA mutations, the virus can't reproduce itself so they insert their genetic material into host cells genome, relying on the host to replicate material. So overtime more copies of the virus infect other cells, and continue the process of embedding instructions for making new viral products in our DNA
HERV: Human endogenous retro virus makes up almost 8% of our entire genetic code
STEM CELLS: Pluripotent cells that could be anything; bone, marrow, neuro, muscle, visceral, blood cells. The genome will eventually tell cells what kind of tissue it will eventually and permanently become
HERV-K: Helps embryos develop a built in immune system, keeping them safe before they develop antibodies to pathogens in the world. Codes for small protein receptors helping HERV-K make viral copies of proteins to infect other cells. Triggering embryonic cells to start making antiviral proteins, building one of the 1st defense against other viruses. After a week of fertilization, the virus leads the placenta to secrete a protein that binds it to the embryo and keeps them attached for next few months of development
HERV-H: Multiple Sclerosis
HERV-K: ALS. Ancient virus
HERV-E: Gives saliva the ability to break down simple sugars, usually performed by amylase from pancreas
GENE: Arrangement of the bases or genetic code 3 letter word that translates proteins
VIRAL RNA: Isolated TX with enzymes to extract or
Serology: Antibody blood test
PCR (Polymerase Chain Reaction): Strand of DNA amplifying or making more copies of DNA. Heat added and proteins breakdown (separate or denature) into single strand of DNA. 2 primers bind, elongates to target viral RNA sequence. Taq Polymerase extends synthesis of new DNA and continues the cycle of copies. Exponential growth = 2 rounds = 4 strands, 3 rounds = 8 strands, 4 rounds = 16 strands, 5 rounds = 32 strands for about 35-40 cycles in the lab
Thermos Aquaticus: PCR technology based on a bacteria that is able to live in hot geyser at Yellow Stone, because with heat it is continually copying DNA with its Taq Primer or DNA polymerase
DNA Polymerase: Add nucleotide to the parental DNA synthesis that continuous and in pieces
DNA ligase: Links pieces together into single DNA strand
DNA Replication: Proceeds in 2 directions (3,5) at many sites simultaneously

Inflammation
Symptoms of Inflammation:
1) Warmth
2) Redness
3) Swelling
4) Pain
5) Decreased Function. Preventing the spread of damaging agents. Dispose cell debris and pathogens. Alerts adaptive immune system. Set stage for repair
Stages of Inflammation: Histamine
1) Vascular change in vesicle constriction and dilation of arterioles
2) Cellular exudate
3) Tissue repair
Immunities:
1) Innate (born with/natural/native)
2) Acquired (after birth)
3) Active Acquired (immunization)
4) Passive Acquired (antibodies) surveillance for pathogens, marks it w/ antigen (flag)
Plasmid: Antibiotics
Autoimmune: Antibodies attach to the TSH receptors, causing an over production of T3 T4, destroying thyroid tissue
Leukocytes: White blood cells, WBCs (10,000-12,000)
1) Neutrophils (60-70%)
2) Lymphocytes (20-25%)
3) Monocytes (3-8%)
4) Eosinophils (3-4%)
5) Basophils (.5-1%)
NK cell: Macrophage. Police with apoptotic cancer cells

Artemisinin
Make machine making machinery. Yeast equipped with genes, regulates processes needed for a whole new metabolic pathway or sequence of biochemical reactions or metabolic engineering. Bacteria and yeast engineered to green fuels (H+ and ethanol) and biodegradable plastics, made with no oil
JVCI: Well established chemical methods to build an entire genome from DNA, based off that of a naturally occurring bacterium. Mycoplasma mycoides, took a cell closely related bacterium, extracted DNA and inserted artificial replacements "booted up with modified" cells as if they were computers with new operating systems. They worked just as well with the modified verified bacterial cells that can be fitted with new instructions that might be stripped down simplified versions of natural "minimal" versions of mycoplasma bacterium
Synthetic Plastic: No organism evolved to digest plastic and it could be there for thousands of years. No decompose and bacteria slowly evolving to feed on it, exploiting energy embodied in polymers (hydro carbon bonds). Vats of modified yeast are able to turn sugar to ethanol
Polymers: Long repetitive molecules primarily made of carbon, shaping and giving plastics their plasticity, or the ability to be molded into any shape. Naturally derived plastics, found in horns, skin, hair and wool, are made with keratin, a mixed carbon and nitrogen polymer. Rubber and wood, are made with cellulose, the polymer provides tough walls of plant cells
Monomer: Carbs/sugars. Repeatable small molecules
Dimer: 2 monomers joined
Isomer: Molecule with same formula

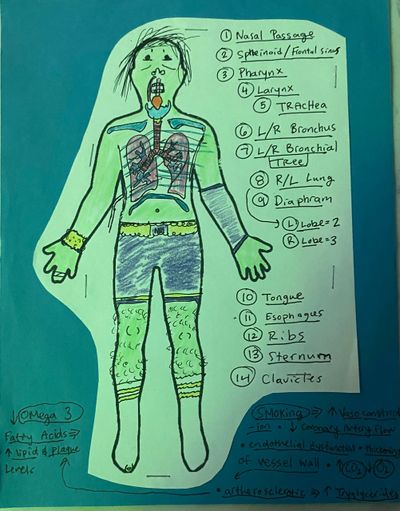
Systems
1) Endocrine (Chakras)
2) Respiratory
3) GI (digestive)
4) Reproductive
5) Integumentary
6) Muscular
7) Nervous
8) Cardiovascular
9) Lymphatic
10) Urinary
11) Skeletal
Organ System: Groups of tissues that specialize for a specific function. Collection of live and dead cells
Tissues: Groups of cells with same function. Surround the cell
1) Epithelal: lines organs that form glands, thyroids, pancreas, skin and lining of mouth and stomach
2) Mesoderm: Smooth muscles. Asthma, Kidney
3) Endothelium: Vascular permeability. Coagulation. Anti-Coagulation. Regulation of vascular tone. Immune. Formation of new vessels by vasculogenesis and angiogenesis
Connective Tissues: Non-living and living tissues with a degree of vascularity. Fibers that need to be cooked (denatured) to breakdown. Connects 1 part to other. Supports. Cushions. Insulate. Bones, blood, cartilage
Skeletal System: Bones (living cells surrounded by nonliving extracellular material) Framework/protection/warehouse. Minerals
206 Types of Bones:
1) Sesamoid (developed in tendons, kneecaps, vertebrae)
2) Long (femur)
3) Flat (Ribs)
4) Short (carpal)
68 Types of Joints:
1) Cartilaginous (slightly moveable connected with cartilage, connective tissue of tightly packed cell (collagen) ribs, pelvic, torso
2) Synovial (move) Ball/socket. Hinge. Saddle. Ellipsoid. Pivot. Glide
3) Fibrous (immoveable/slightly connected by tense connective tissue. Skull sutures, forearm
Stored in bones:
1) Ca+
2) Mg
3) Ph
Yellow bone marrow: Stores fats
Red Marrow: Eventually turns yellow but is there for RBC production
Circulatory System:
1) Blood vessels (delivers O2 and nutrients)
2) Carry away waste
3) Maintain BP
Arteries, Veins, Capilaries, Capules, Venules
Lymph: Endothelial cell ducts "Thymes" (R drains to internal jugular) (L drains into thoracic duct) Subclavian vein
Peyers Patch: Distal of the small intestine. Part of the lymph system
Tissue Damage: Caused by chemical mediators (histamine, serotonin, prostaglandins) to open Na+ channels
Endocrine/Exocrine System: Maintains homeostasis with slower moving hormones through blood that targets broad areas and have lingering effects chemically. Most hormones are amino acid based steroids, synthesized from cholesterol
Hormones: Long distant signals. Lymph and blood
1) Amino acid based
2) Steroids
3) Eicosanoids
Pineal Gland: Pareital or 3rd Eye, named after its pine cone shape, secretes the hormone melatonin. Melatonin helps synchronize circadian rhythms in different parts of the body. Circadian rhythm is your 24 hr. internal clock, responding to light and darkness, releasing more melatonin when it’s dark out. Acting with the hypothalamus, it alarms time to slow down activity for the day. While also protecting neurodegeneration and regulating menstrual cycles. Aligning with our chakras to give spiritual insight, intuition and consciousness. A lightning rod of the soul. Buddhist metaphysics state DMT, the spiritual molecule, secreted from the pineal gland enables transit of life force into this life and from this life to the next
Androgen: Group of hormones like testosterone (primarily influence the growth and development of male reproduction system
Pituitary: Produces growth hormones
1) Estrogen
2) Progesterone
3) Testosterone
Anterior Pituitary: Produces TSH
Thyroid Gland: Need iodine to work
Parathyroid: PTH (phosphate and calcium levels)
PTH: Goes to the
1) Bones
2) Kidneys
Bones send Ca+ to blood vessels. Kidneys also send Ca+ to blood vessels and Vitamin D in intestines sends Ca+ to blood vessels
Pancreas:
1) Insulin
2) Glugagon (muscles burn to heat up)
Alpha: Secretes glucagon
Beta: Secretes insulin
Insulin: Pancreatic hormone that regulates storage of glucose
Delta: Secretes soma statin
Adrenal Cortex: Stress
ADH: The more of it, the more water reabsorption in renal tubules (increased intravascular fluid volume) Dilutional hyponatremia and decreased serum osmolality
RAAS: Adrenal (Kidneys the doors to the club)
Na+ (Girls)
H2O (Guys)
Renin (CEO and can tell them to shut down club kidneys)
Angeotensin 1 and 2 (Assistant Managers, says to hold Na+ in: wants a bunch of girls at the club to entice H2O too)
ACE Inhibitor (Cops, that cuts off the line of communication and can shut down the club kidneys)
ARBS (blocker, like the pimp who allows the Na+ to leave 1 and then H2O to follow
Aldosterone (Bouncer at the door, enzyme made in kidneys, told by assistant manager (angiotensin) to shut down but still let in Na+ and no fluid is released from the body
Powered by GoDaddy
Cookie, cookie, cookies start with C!
Guess that the only way GoDaddy can tell me if anyone actually came to see the site, is with the cookies. Thank you for being here and amazing, I appreciate you